Macrocyclic small molecules have attracted significant attention in drug discovery over the past decade. Drawing inspiration from complex cyclic natural products, macrocyclization of bioactive small molecules has been demonstrated to increase their potency, target selectivity, and other biological and physiochemical properties. In addition, (semi)peptidomimetic macrocycles are capable of tackling previously “undruggable” biological targets.[1][2][3]
Methods for macrocyclization
Macrocyclization of precursor molecules can, in theory, be achieved under a range of chemical coupling conditions. However, the success of this reaction is greatly dependent on the ring size, linker compositions, and handle orientation. Focusing on the most strained classes of medium-sized rings and macrocycles (9–16 membered), at Symeres, we have developed a synthetic toolbox, which comprises optimal conditions for several different ring sizes and linker geometries.
Chemical diversity in macrocycles
For larger macrocyclic molecules, another challenge lies less in the cyclization itself, but rather in their lengthy synthetic routes and the extensive effort it takes to introduce molecular diversity. At Symeres, we have extensive experience with such projects, in which a modular approach allows for the generation of large sets of chemically diverse fragments that can rapidly be assembled – in a parallel fashion – into the corresponding macrocycles.
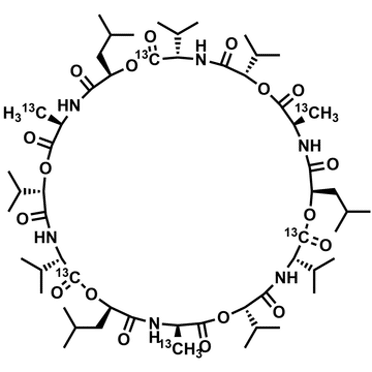
A relevant example of a large synthesized macrocyclic molecule is Cereulide[4], a toxin produced by some strains of Bacillus cereus. It has been synthesized with six 13C-labels[5] at Symeres and is offered for sale by Chiralix[6].
Structural analyses of macrocycle conformations
It is well established that conformational preference and the stability of macrocycles are essential for their potency. We have found that including both solution-phase and solid-state structural information at an early stage in medicinal chemistry projects helps to focus compound design, and thereby, speeds up the optimization process.
Contact us via the form below for more information about our capabilities.
[1] J. Mallinson et al., Bioorg. Med. Chem. 2012, 20 , 1409 DOI: https://doi.org/10.4155/fmc.12.93
[2] J.A. Amrhein et al., J. Med. Chem. 2021, 64 , 7991 DOI: https://doi.org/10.1021/acs.jmedchem.1c00217
[3] P.G. Dougherty et al., Biochem. J 2017, 64 , 474, 1109 DOI: https://doi.org/10.1042/BCJ20160619
