Symeres has considerable experience and a proven track record in the synthesis of complex carbohydrates and related molecules. This specialized branch of synthesis can be challenging, due to polarity, chemical complexity, the lack of a chromophore, and, in many cases, the need for complex protection strategies for the target carbohydrates.
Below are some examples of the kind of challenges that Symeres is used to addressing.
Glucuronides of active pharmaceutical ingredients (APIs) are important tool compounds in the investigation of drug metabolism. SN-38 glucuronide is a metabolite of irinotecan, a topomerase inhibitor used for the treatment of various forms of cancer. 3-Desmethylthiocolchicine-3-glucuronide is a metabolite of thiocolchicoside, a muscle relaxant with anti-inflammatory and analgesic effects.
Diosmetin-3’-O-glucuronide and diosmetin-3’,7-di-O-glucuronide are metabolites of diosmin, a dietary supplement used to aid the treatment of hemorrhoids and venous diseases.
Benazepril acyl-β-D-glucuronide is a metabolite of benazepril, an ACE inhibitor used for the treatment of hypertension and congestive heart failure.
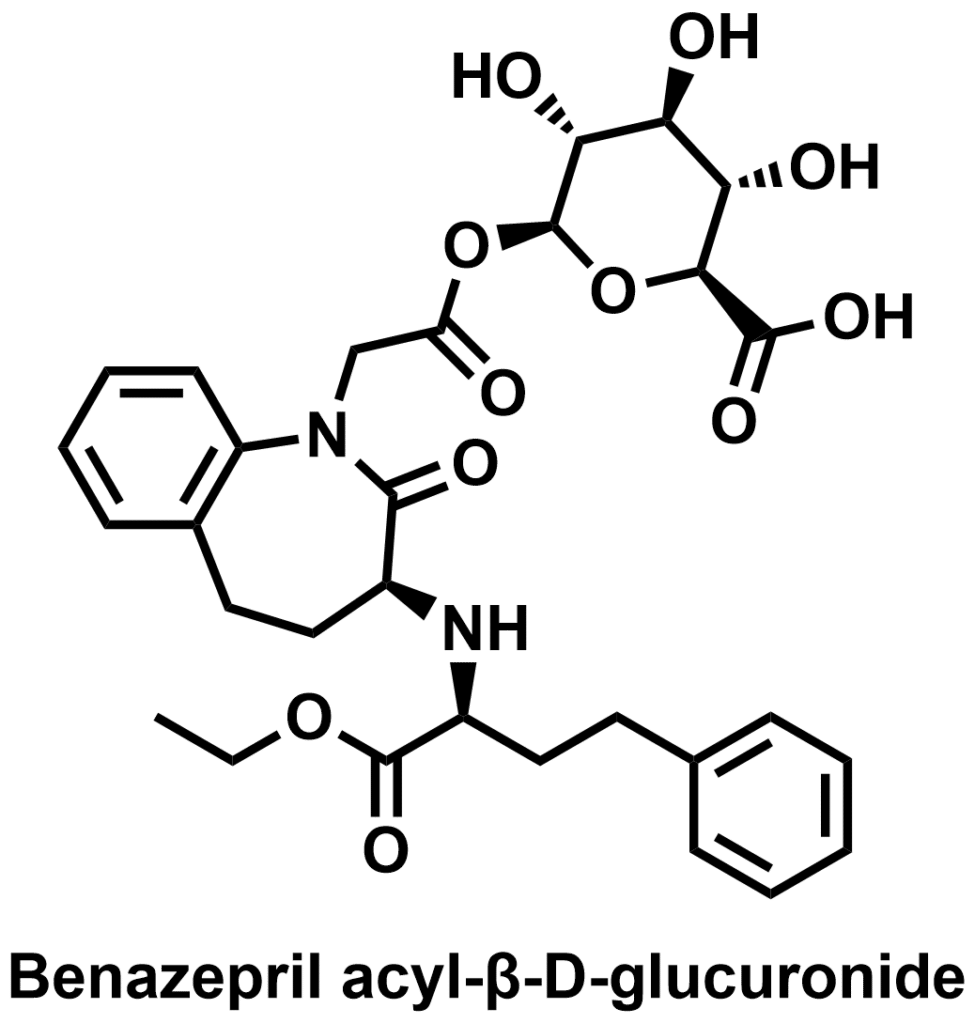
All glucuronides depicted are available from our Chiralix catalog.
The main challenge of glucuronide synthesis is typically to find the right conditions for coupling of the activated glucuronide moiety with the substrate at hand, mild removal of the protecting groups, and careful chromatography to obtain the desired glucuronide product in high purity.
Another type of carbohydrate chemistry is centered around the preparation of disaccharides or tri-, tetra-, or higher oligosaccharides. This involves classical carbohydrate methodology and having the appropriate protecting groups in place to join the building blocks together in the desired fashion.
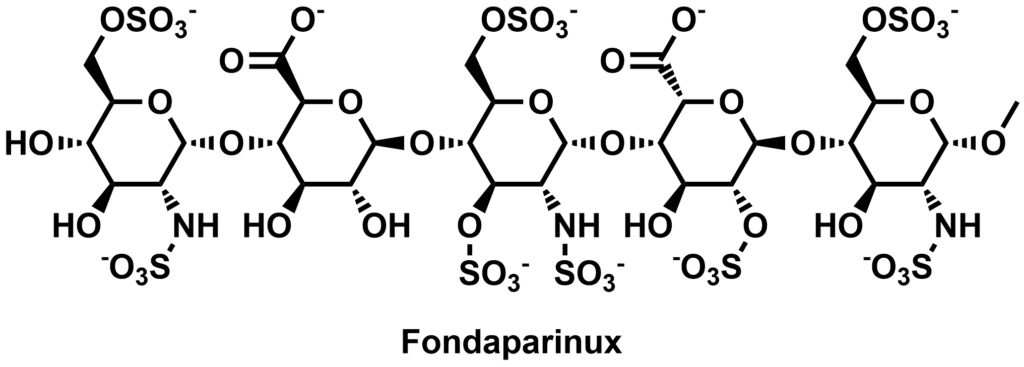
Fondaparinux (Arixtra), a pentasaccharide anticoagulant with 25 chiral centers, is a classic example. Its chemical synthesis comprises over 60 reaction steps. Various impurities, in which one of the many chiral centers was inverted, were identified and synthesized at Symeres. Substituting one sugar moiety for another may look trivial but is far from it from a synthetic point of view, as the various sugars (glucose, galactose, idose, etc.) exhibit very different reactivities.
Contact us to learn what we can do for your carbohydrate chemistry!
