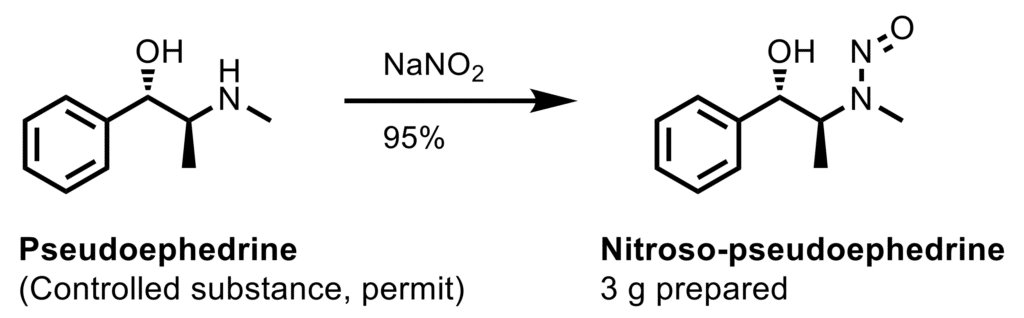
Symeres has a dedicated group of chemists for the synthesis of reference standards to support our clients working in chemical development (CMC) and GMP production of APIs. For example, the synthesis of (i) impurities and decomposition products formed in API production, formulation studies, and storage of the drug substance or drug product; and (ii) analytical markers like (cold) labeled compounds (deuterated and 13C-labeled APIs, link to section labeled compounds Mark Verhaar). Examples include oxidized, hydroxylated, or hydrolyzed APIs with typical functional groups including N-oxides, hydroxylamines, and nitrosamines (see scheme).
As well as synthesis, the isolation of impurities from process streams or formulations by preparative HPLC/SFC, followed by elucidation of the molecular structure by advanced NMR techniques and mass spectrometry is routinely carried out. Ultimately, the structure of the impurity is proved by synthesis, HPLC spiking experiments, and material supplied as a reference standard for CMC purposes.
Contact our experts directly via the form below!
